Understanding the German health care market and how its participants are wired
Small-group immersion course for medtech companies and investors
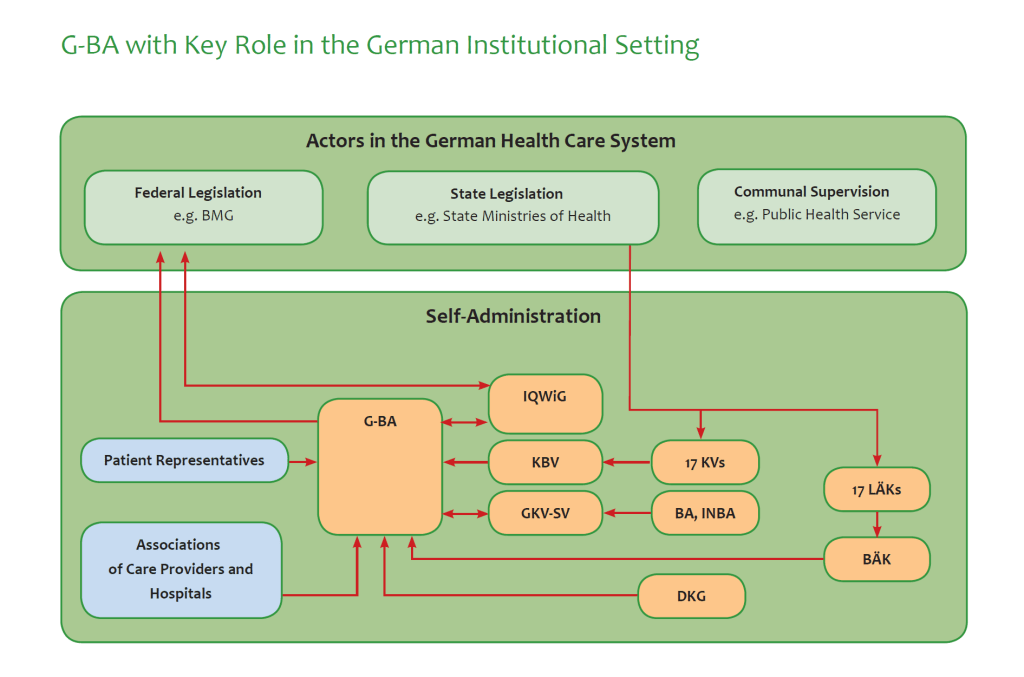
With a total volume of around EUR 480bn, Germany is a large and attractive health care market. Like most other mature systems, the financing and provision of health is highly complicated, ever-changing and massively influenced by special-interest groups. The duality of social and private health insurance, the crucial role of the Joint Committee, the importance of data protection, the governing function of the Ministry of Health, the inherently private for-profit nature of most providers and the importance of corporate co-operatives managing the self-administration are some of the key elements in this system.
How to access the 480-bn-Market:We at the Medizin-Management-Verband can provide foreign companies with a comprehensive overview of the German system and help answer the following questions:
Looking from outside the health care market seems very much a closed shop. And in fact, the first health care market of Germany, around 280 bn Euro per year funded by the Statutory Health Care Insurance System (SHI), is not a “market” at all, but a regulated system to its full extent: Complex regulations, semi-transparent government agencies and fierce lobbies are interacting in a mix of traditions and networks.
Entering the SHI SystemWe show you the formal and informal machinery, the stakeholders, risks and potentially fruitful strategies to enter this regulated system. We can provide the pathways to enter new products and services into the authoritative listing of items paid for in the SHI system

Lots of legal pitfalls and traps are lurking along the long and windy roads of the health care system. Data protection, the law on marketing of drugs and medical devices, the German law against unfair competition and many other tricky regulations have torpedoed promising projects. To know these obstacles helps to avoid them.
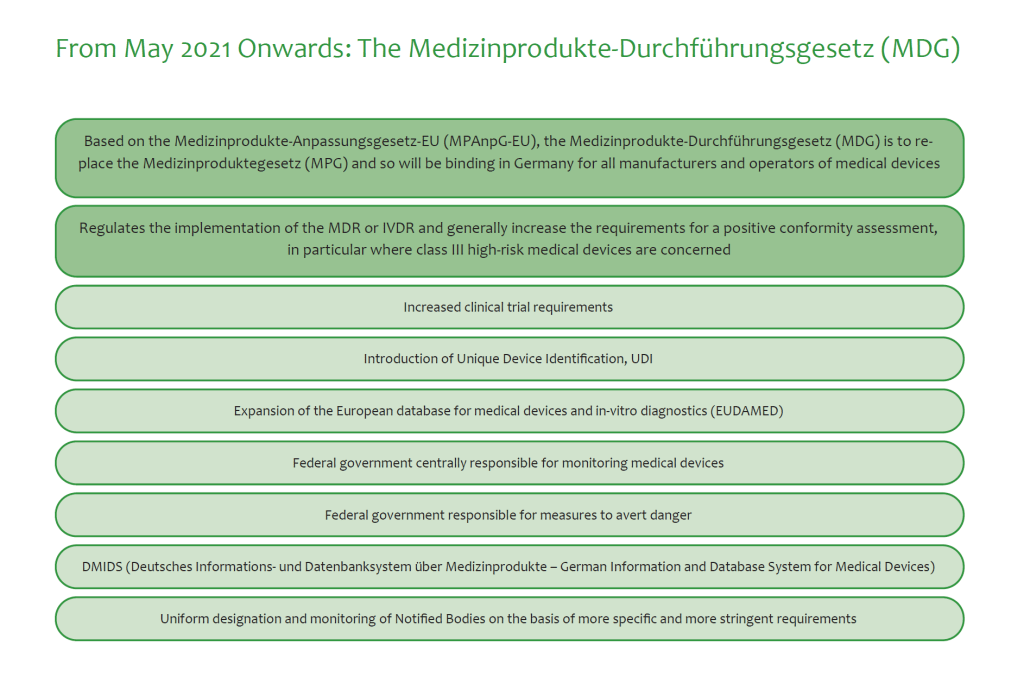
Getting medical devices registered and listedUsually, it takes European Union’s CE approval depending on the kind of device. This process is in transition right now from the national law on registration of medical devices to the European Medical Device Directive (MDD).
Many studies rank the German healthcare system low regarding digital options. This is likely to change as digital health regulation is on the rise. The Digital Care Act of 2019 (“Digitale-Versorgung-Gesetz”, DVG) introduced coverage for medical apps (“Digitale Gesundheitsanwendung”, DiGA).
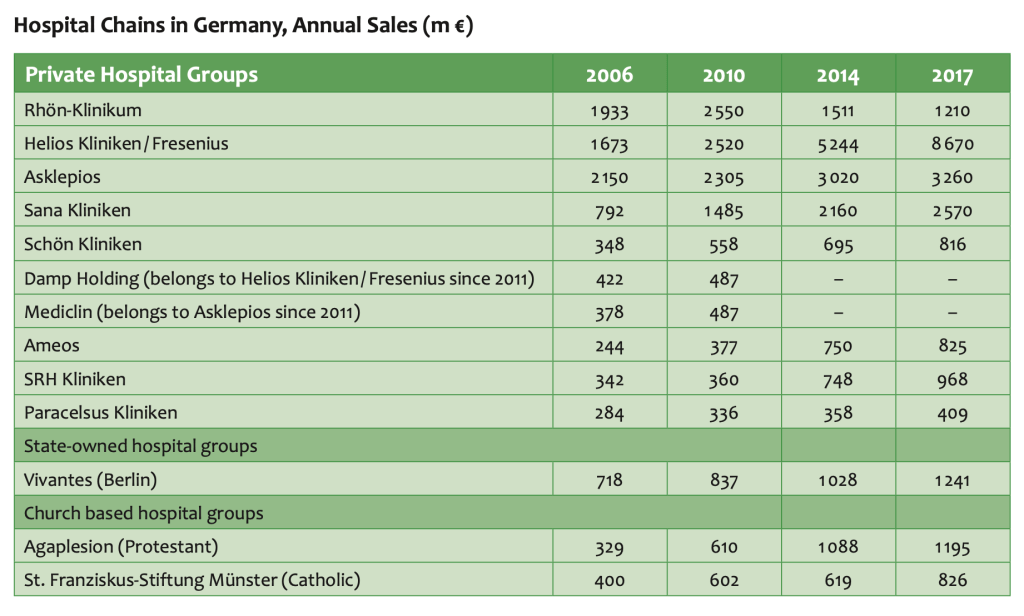
Business with the big providersIn inpatient care market is subdivided in a net of large privately owned, modern hospital chains, university-based entities, public hospitals and confessional organizations. We explain recent market developments, the flow of investments and reimbursements, and key decision makers.
The outpatient care sector is provided for by an inhomogeneous set of some 250.000 independent ambulant medical care providers. How are decision made at the level of the individual provider? Where to find current and future key opinion leaders? How to gain access to them? This might range from elaborate communication concepts to setting up a sales force.
Target audience:CEOs, product managers, brand managers, marketing specialists; group workshops possible.